Happy Labor Day. Not sure what that’s supposed to mean, really. Perhaps – be happy that you are not being forced to work on this day. So the greeting applies only to those not working shifts or in many service industries or in other blighted corners of the economy where workers are working because they have to and are most decidedly not happy about it. Fine.
I recently hosted for P2, an Artificial Intelligence / Machine Learning / Self Driving Labs (AI/ML/SDL) workshop at the University of Toronto in partnership with the recently formed and generously financed, Acceleration Consortium there. My opening remarks went like this: I have a newspaper cutting in my study that talks about my great-grandfather who went down the pit (coal-mine ) at the age of 9 to get coal out of hard to reach pockets. He managed to raise a family despite the less than optimal work-life balance. His son, my grandfather, lived then in more enlightened times and went down the pit at age 13. He also managed to raise a family and my father avoided the pit altogether. He went into an office at age 16 and was working when the weekend as a widely accepted practice was adopted by his employer, thus giving him every Saturday off. It was a great day in our house. The garden never looked so good and the family never went on so many outings and picnics and whatnot. Me? I’ve never been down a pit although I’ve worked plenty of Saturdays – but not because anyone told me to.
What powered this transition from Dickensian to Dignity in a few generations? Revolutions: The energy revolution, industrial revolution, computing revolution, data revolution, social revolutions. The next revolution is the Intelligence revolution, meaning the price of intelligence is getting cheaper. In the same way that mechanical innovation replaced 9 year olds in the pit, and got the coal out of there, faster, cheaper and more safely and completely, AI can alleviate millions from drudgery and do things similarly better and cheaper. Do this right and humans as individuals and a species can focus more on fulfilling their potential for wisdom and spiritual enlightenment, if they so choose. Someone in the workshop (frankly I wish it was me, but it wasn't ) coined the phrase “Everything is chemicals / Chemicals are everything” and so why shouldn't we be looking to make them better, faster, cheaper, safer and more sustainably with the aid of technology. That was the aim underpinning the workshop which pulled together leaders in industry, consumer goods, finance and academia for a pre-competitive planning session, the results of which should, if pursued right, bring the benefits of a happy Labor day to many more.
Two other thoughts on Labor Day. First, I like it better than May Day, which we had in Europe. May Day, to me, was too closely aligned with a movement which killed 10’s of millions of people through coercion. Not a good look for labor. Second, I went to high school in a town with a number of private schools each with their own, usually Latin, motto. I remember one in particular Labor Omnia Vincit – Work conquers all. The dour slogan matched by the dark earthen-brown of the uniforms. I genuinely felt sorry for those kids as I imagined them grinding in cowed silence through their lessons under the humorless watchful eye of camp-guard teachers. Our own Catholic boys’ school’s motto was the less foreboding Deus Lux Nostra – God our light; matched by a much cooler black and gold uniform. And while we no doubt labored under equally humorless overseers, it seemed OK because, well, God was our light and so it really didn't matter, did it? Of course the ultimate school motto in my view, was Amor Omnia Vincit – Love conquers all. A delicious middle finger to the work-conquers-all regime! Those kids surely had it made. No need to work, just love all day. In 70’s teenage boy epistemology, love differed not at all from sex. It took me a few more years to realize that, love needed work to make it last and work needed love in order to really do your best.
In Matthew Chapter 11, Jesus notes “Come to me all who labor and are burdened and I will give you rest”, implying that the opposite of labor is rest or the antidote or cure for labor is rest. Seems about right. But then what about love? Well, if you accept that the alleviation of another’s burden is an act of love, then the “cure” for the burden of labor, if you like, is love. So, yeah work gets stuff done, no doubt and it may indeed conquer all in the secular realm. But, by alleviating the burdens of work, love actually conquers all, overall. Happy Labor Day!
August was a very light news month in the world of surfactants and so I’m going a bit further afield to glean some interesting nuggets for you to read:
Mid-cut fatty alcohols enjoyed (?) a quiet summer as prices edged down on weak demand. US prices for 1215 alcohols stayed around $1,645 per MT according to analysis by ChemAnalyst.
In the US ethylene oxide market, some activity. Following the Dow, Plaquemine explosion last month, the EO unit is expected to be down for at least 6 months according to the company. Despite the market shortage of EO, prices have ticked up only modestly due to depressed demand. According to OPIS, a Dow Jones company, The Plaquemine site covers 3,300 acres and includes 23 manufacturing plants, including two olefins units with annual ethylene production capacities of 1.245 billion lbs and 2.35 billion lbs, along with several polyethylene units that produce linear low-density polyethylene and low-density polyethylene grades. The two EO-focused units at Plaquemine consume an estimated 233,000 metric tons per year of ethylene from the site's olefins plants. On a daily basis, this is a total of about 1.4 million pounds of ethylene. If only Glycol 2 is shut, this consumption figure is reduced by half.
A big shout-out is overdue here to the AOCS (American Oil Chemists Society) who put together an impressive series of events and publications, including the great INFORM. The September Issue is all about surfactants. No reader of this blog has any excuse for not being a member of AOCS and having access to this journal. The issue covers, among other things, the use of computational platforms that analyze and predict the toxicity of chemicals. There’s a wealth of data, spanning decades of research, on the biodegradability and toxicity of various compound that is now being used in new chemical research in a computational framework. Super- cool. Also in the September edition, an article by George Smith (yes, the George Smith) about bio-surfactants. Worth a read.
Also there’s a great analysis in INFORM of the revised European regulations covering detergents and surfactants. Hey, hey, don’t skip this bit! I know, that even I have been guilty in the past, of calling regulatory stuff boring. I was a mere youth back then. Intelligent but not yet wise. Now armed with the benefits of both intelligence and wisdom, I see the bigger picture and, as INFORM so clearly declaims, it’s this: The detergents industry accounts for approximately 4.2% of the production value of the total European chemicals sector in 2018. The total market value of the European detergents industry in 2020 was € 41.2 billion. The manufacturing of products for the whole market that includes both consumer and professional products involves around 700 separate facilities throughout Europe. Regulation (EC) No 648/2004 of the European Parliament and of the Council of 31 March 2004 on detergents (‘the Detergents Regulation’) lays down the rules that detergents need to comply with in order to be placed on the EU market. The revised rules cover new innovative products like detergents containing micro-organisms and new practices like the refill sale of detergents. The new rules also introduce a digital labelling and a product passport for detergents and surfactants. I found this to be particularly interesting and so I went to the source document from the EU (thankfully still published in English) here.
Here is a snippet direct from the EU proposal: “To make this regulation future-proof, this proposal replaces the EU declaration of conformity set out in Decision 768/2008/EC with the obligation for detergents and surfactants to have a product passport demonstrating compliance with the requirements of this Regulation. The product passport will be connected through a data carrier to a unique product identifier, and meet the same technical requirements for a product passport under the ecodesign for sustainable products regulation27. The reference of the product passport must be included in a Commission central registry that will be set up under the ecodesign for sustainable products regulation, and this information must be presented at customs.”
I actually kinda like this digital passport idea. Here’s another thought that springs to mind. The use, overuse or misuse of surfactants and detergents has killed how many people? 8 in the last 10 years. According to Wikipedia. OK. And the number of deaths from the use, overuse or misuse of sugar? 184,000 (per year!!) according to analysis by Mozzafarian et al in the journal Circulation. Let’s say I’m off by a factor of 100 in each direction. Still not even close. And, yes of course, there is the environmental impact of detergents that the regulations are seeking to address. Environmental damage is the classic case of the public cost of private profit. The sugary-drink industry is another such case where the companies that design, make and market addictive products to kids enjoy nice profits. The costs associated with those profits however, are spread across the public, first to the direct consumers whose lives are cut short, and then more broadly to society that bears the medical costs of care and the costs of the foregone human potential of the sick and the dead afflicted by these products. Think about it. Private profit and public cost is a deadly combination – in any industry. Oof – end of sermon.
Something else. Readers have probably figured out that I’m a bit besotted at the moment with AI. So.. what about AI and surfactants. Anything there? A bit for sure, but not as much as there will be in the coming 5 years and I personally want to experience and even drive as much of that as I can. Stay tuned, here, for more in the coming weeks. So – anyway, AI and surfactants – a non-representative sampling of cool things - with footnotes and all. No extra charge..:
One of the first studies that demonstrated the potential of AI in surfactant synthesis was published in 2002 in the Journal of Physical Chemistry[1]. Neural networks were used to predict the surface tension of surfactants based on their molecular structures. Cool tool for surfactant design.
More recently, The Journal of Chemical Information and Modeling[2] in 2019, reported that artificial neural networks were used to optimize the synthesis of bio-based surfactants. Neural networks were able to identify the optimal synthesis conditions, to make highly efficient and biodegradable surfactants.
In the area of surfactant applications, A study, published in the Journal of Colloid and Interface Science[3] in 2011, used genetic algorithms to optimize the composition of surfactant mixtures. The algorithms were able to identify optimal surfactant mixtures with improved properties, such as lower surface tension and higher stability; suggesting new applications for surfactants in cosmetic and personal care, the oil and gas, food and other area.
Deep learning algorithms, such as convolutional neural networks (CNNs), have been used to predict surfactant applications. In a study published in the Journal of Chemical Information and Modeling[4] in 2018, CNNs were used to predict the critical micelle concentration of surfactants based on their molecular structures, providing a promising approach for the design of new surfactants.
In a nuanced analysis published in the Journal of Physical Chemistry[5], the authors ask Can machine learning predict the phase behavior of surfactants? With the data set used (which is the most comprehensive openly available one for this type of study to the best of our knowledge), the answer is “yes”, but the predictions can be improved. The authors identified three areas in which data provided to the ML classifiers warrants further development, i.e.: improving the data bias in surfactant phases observed, building better training data with respect to the chemical space (i.e., different surfactants) and providing more comprehensive feature space (i.e., relevant chemical characteristics).
I know this is getting rather academic, so here’s a press release from a company called De3.ai. I have literally just stumbled across them and so imply no endorsement or otherwise via their inclusion in the blog. Apparently, De3.ai and Biosurfactants LLC plan on building a Rhamnolipid Biosurfactant facility in Tampa, FL. Using artificial intelligence algorithms, De3.ai can determine the exact application to eliminate contaminants and microbes from environments with no carbon footprint and no residual effects on the environment after application. Rhamnolipid Biosurfactants are chemicals secreted from bacteria that have many applications including breaking down the cell walls of pathogens. Interesting. Let’s see.
There’s more. In 2006 the Society of Petroleum Engineers[6] published an analysis showing the use fo AI to predict the surfactant loadings needed to optimize enhanced oil recovery.
And more: A pre-publication on the SSRN (Social Science Research Network) website[7] concludes that Chemometrics/Machine Learning methods such as Blind Source Separation (BSS) are powerful tools for extracting signals from complex mixtures. These techniques have been successfully applied to several detergent mixtures for various household applications. The combination of spectral databases of surfactants and mixtures has enabled the identification and quantification of surfactants in these complex mixtures.
There’s a bunch of review papers out there and honestly it’s hard for me to recommend any in particular. There’s an old one from 2018 in Nature[8] that highlights the usefulness of publicly accessible databases that I like.
There’s also what seems like a decent review in the Journal of Chemical Sciences from last year[9]. It’s also free to access here. Some of the figures are quite accessible.
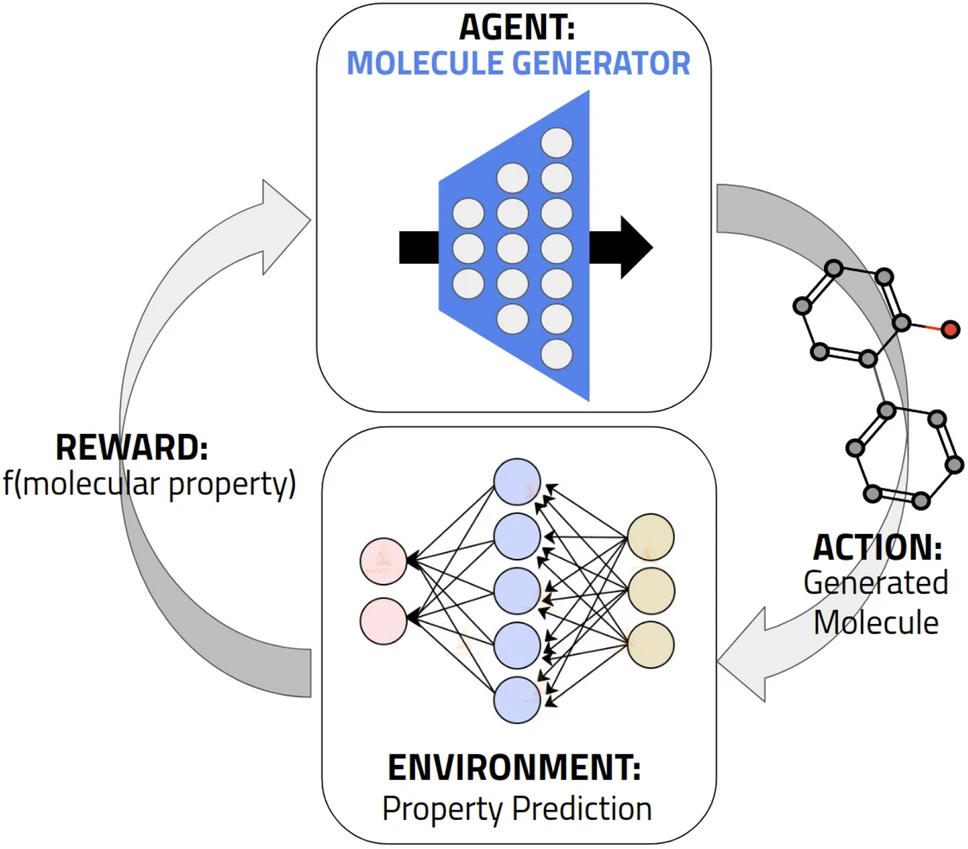
Others not so much
Finally, this slow news month: Researchers at UC Santa Barbara, have reported an improved catalytic method for converting waste polyolefins into alkyl aromatics – for subsequent use as surfactant feedstocks. Thus enabling a potential detergent bottle to detergent conversion. The original paper can be found here https://doi.org/10.1016/j.chempr.2023.05.017 . Very cool. Not close at all to commercial status but intriguing nonetheless. I wish them well.
Music this month? Hmm… well the abovementioned Toronto AI workshop called to mind this classic from, of course, Rush. Electricity? Biology? Seems to me it’s Chemistry. (Notice the cityscape!)
You know, that song, in my mind, always was paired with this one from Rush, from Moving Pictures, the under-recognized Vital Signs. There is so much in this one; an early Rush reggae song – just soak in the guitar and the bass. And the lyrics – for me address the beginning of human life and a precondition for life – deviation from the norm, which they conflate with elevation and escalation from the norm. A prescription for human development. :
That’s it for this month. By the time you read this, the summer will have officially ended in the US (labor Day) and work starts up again in earnest. Often feels more like a new year than January. Have a great year ahead; filled with your own human development - and love.
[1] Wu, X. G. Wang, “Artificial Neural Network Model for Predicting the Surface Tension of Nonionic Surfactants”, Journal of Physical Chemistry B, Vol. 106, No. 11, 2002, pp. 2777-2782.
[2] Chen, L. Zhang, X. Cui, “Optimizing the Synthesis of Bio-Based Surfactants Using Artificial Neural Networks”, Journal of Chemical Information and Modeling, Vol. 59, No. 9, 2019, pp. 4661-4669.
[3] D. A. Stuart, K. L. Yeoh, “Optimizing Mixture Composition of Surfactants Using Genetic Algorithms”, Journal of Colloid and Interface Science, Vol. 359, No. 2, 2011, pp. 607-613.
[4] Zhang, X. Gao, Y. Fan, “Prediction of Critical Micelle Concentration of Nonionic Surfactants Using Convolutional Neural Network”, Journal of Chemical Information and Modeling, Vol. 58, No. 5, 2018, pp. 879-887.
[5] J. Phys. Chem. B 2023, 127, 16, 3711–3727
[6] Weiss, William W., Xie, Xina, Weiss, Jason, Subramanium, Vishu, Taylor, Archie, and Fred Edens. "Artificial Intelligence Used to Evaluate 23 Single-Well Surfactant-Soak Treatments." SPE Res Eval & Eng 9 (2006): 209–216.
[7] Clément, Yohann and marote, pedro and lanteri, pierre and Bonhomme, Anne and Martin, Marie, Artificial Intelligence for Reverse Engineering:Application to Detergents Using Raman Spectroscopy. Available at SSRN: https://ssrn.com/abstract=4510701 or http://dx.doi.org/10.2139/ssrn.4510701
[8] K.T. Butler et al. “Machine learning for molecular and materials science" Nature volume 559, pages 547–555 (2018)
[9] Karthikeyan, A., Priyakumar, U.D. Artificial intelligence: machine learning for chemical sciences. J Chem Sci 134, 2 (2022).